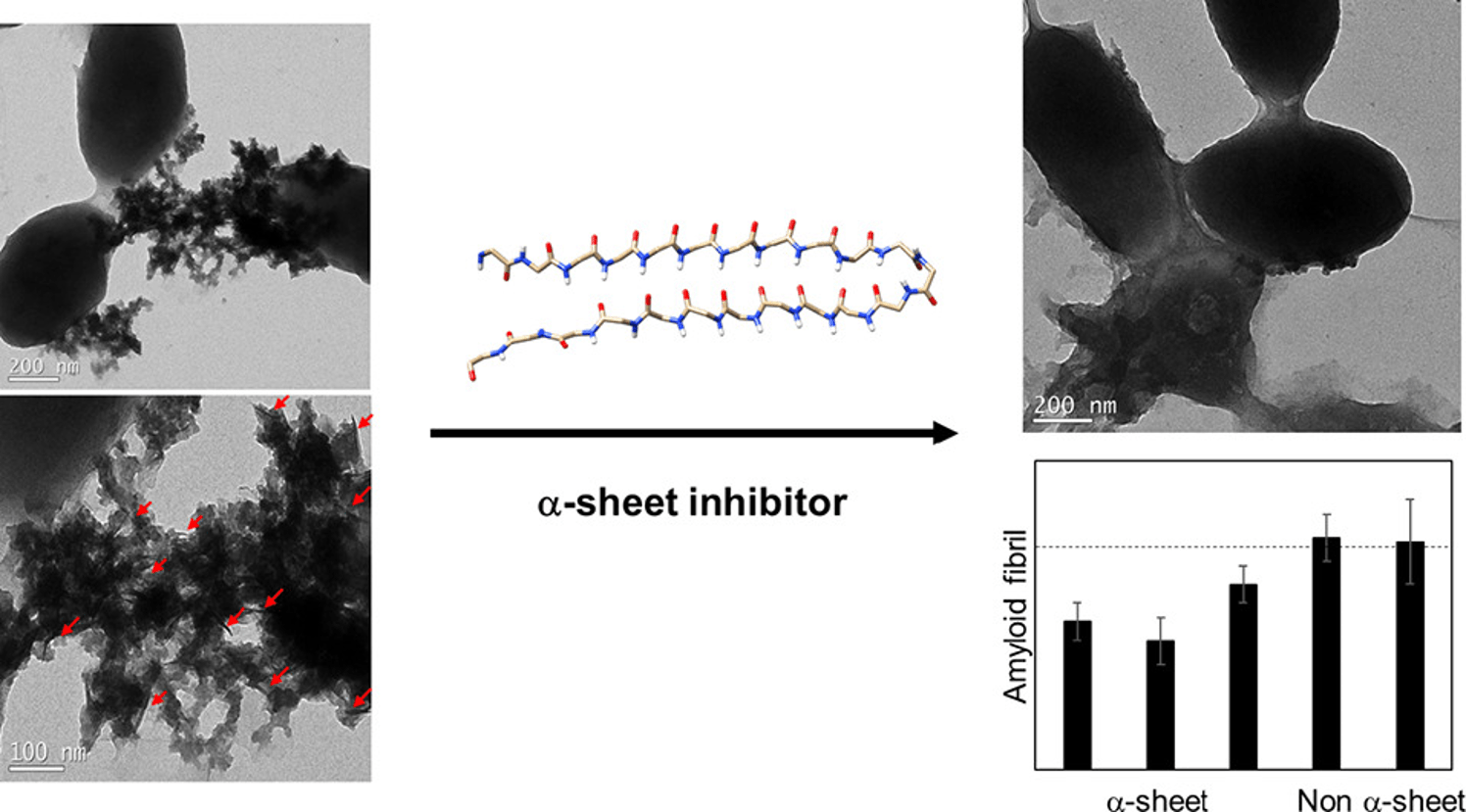
Some proteins are amyloidogenic, aggregating into toxic species. We design proteins with the aim of limiting that aggregation, slowing disease progression. With this goal in mind, we take advantage of the α-sheet secondary structure we observed in many amyloidogenic proteins. By designing peptides (short sequences of amino acids) with α-sheet structure, we found that these peptides preferentially interacted with the toxic intermediates in amyloidogenesis, inhibiting aggregation.